Kangaroo Mother Care: A Case Study on KMC’s Beginnings and its Worldwide Implementation (Part I)
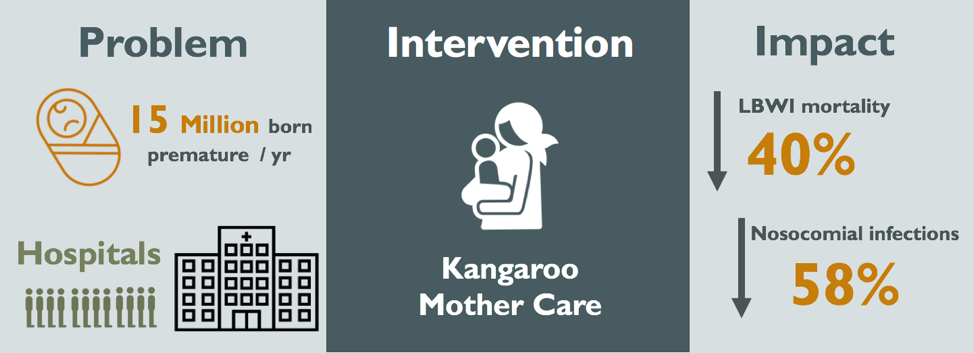 Figure 1: Visual Abstract of the Kangaroo Mother Care Method
Figure 1: Visual Abstract of the Kangaroo Mother Care Method
Editor’s Note: This article was written by Courtney Fang, Zoë Goldstein, Morgan Kennedy, Carla Achcar, Bidjinie Coriolan and Mackenzie Hamilton, for a case study in PPHS 511: Fundamentals of Global Health. Read Part II here.
Abstract
Every year, 15 million infants are born prematurely (WHO, 2017). The majority of preterm infants have low birth weight (LBW), associated with risk of infections and hypothermia in the short-term and impaired cognitive skills and chronic health issues in the long-term. Traditionally, LBW infants required long hospital stays with artificial incubation. In 1978, Kangaroo Mother Care (KMC) was implemented at the Instituto Materno Infantil in Bogotá, Colombia to treat premature infants at a relatively low cost, as the institute was overwhelmed with overcrowding, nosocomial infections and lack of incubators. Since its inception, KMC has been implemented in both developing and developed countries alike. The implementation of KMC was associated with a reduction in risk of mortality by 40%, nosocomial infections by 58%, and savings of approximately $570 USD per patient (Conde-Agudelo, 2014; Sharma, 2017). While these results are hopeful, there have been implementation difficulties in African and South Asian countries. Moreover, most research has been reported on facility-based KMC, in stable infants. More research must be conducted to evaluate potential use of KMC in unstable infants as well as to evaluate community-based KMC, since home deliveries are common in many settings. Here, KMC could potentially have the most impact on reducing premature neonatal deaths.
Introduction
Where it all began…
The KMC program originated in the Instituto Materno Infantil in Bogotá, Colombia in 1978, where an estimated 11% of babies were born weighing 2.5kg or less (Charpak, 2001; Simkiss, 1999). Pediatrician and department head Dr. Edgar Rey Sanabria invented KMC to address the overwhelming number of high risk, LBW infants born in his maternity ward each year (Charpak, 2005). At the time, skin-to-skin contact was not a cultural practice and the Instituto Materno Infantil was run down with overcrowding, delivering roughly 100 babies per day (Charpak, 2017). Of these infants, 30% were LBW (Charpak, 2017). The hospital was also burdened with nosocomial infection, lack of incubators, and frequent infant abandonment (Charpak, 2005; Simkiss, 1999). With these high-risk infants double-bunked and separated from their mothers, infection outbreaks were common and the mortality rate unacceptably high (Simkiss, 1999). Additionally, an alternative was needed to the expensive infrastructure and highly skilled staff demanded by the existing medical care. To successfully combat this, Dr. Sanabria modeled Kangaroo Mother Care specifically for underserved environments without high-quality neonatal technology (Charpak, 2005).
The main goals of this intervention were to improve the prognosis of LBW infants. Additionally, the treatment aimed to encourage close relation as soon as possible after birth between mother and child, and humanize the in-hospital and outpatient care of premature babies.
Our Case Study
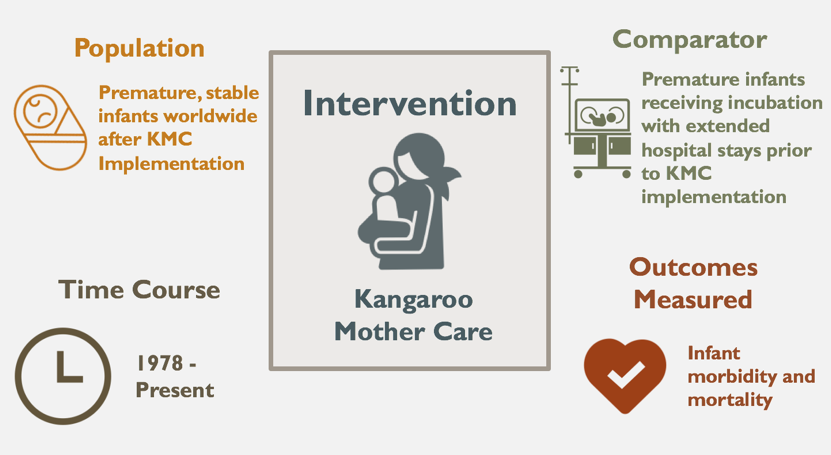
Since its inception over four decades ago, KMC has been implemented in both developing and developed countries alike (Ruiz-Pelaez, 2004). This study analyzes data from various time points from KMC’s first implementation in Bogotá to its present implementation globally, evaluating its impact, cost-effectiveness, and feasibility in future implementation.
What is Kangaroo Mother Care (KMC)?
Kangaroo mother care (KMC) is a scientifically proven method consisting of skin to skin contact between the mother and the infant, exclusive breastfeeding, and early home discharge in the kangaroo position (Charpak, 2005). As marsupials, kangaroo mothers give birth to their infants earlier than other mammals. Before their offspring can exist on their own, kangaroo mothers care for their children in a pouch external to the womb (Hall & Kirsten, 2008). Similarly, KMC for humans allows for the continued nurturance of the preterm baby even after birth. In the kangaroo position, the infant is held vertically with direct skin-to-skin contact between the mother’s breasts (WHO, 2003). Both the mother and baby are wrapped in a secure binder with their chests touching (WHO, 2003). The infant’s head is laid to one side and turned slightly upwards to allow for unobstructed breathing (WHO, 2003). Moving the baby from the binder is generally avoided, even for bathing. Exceptions are made for transitioning the baby to another caregiver’s chest, such as the father’s. It is necessary that the kangaroo position be uninterrupted and sustained until the gestational age of 40 weeks (WHO, 2003). These protocols aim to help premature LBW infants regulate body temperature and improve weight gain. The mother acts as the main source of incubation, stimulation and feeding. The process typically begins in a hospital and adapts to home-care with regular check-ups in a KMC clinic.
Although infants need to be stable before being held in this position, LBW preterm infants are unable to regulate their own temperature (Charpak, 2005). As previously mentioned, hypothermia is one of the primary causes of neonatal death. KMC ensures that the mother helps to regulate the infant’s temperature through her own body heat. The intervention is discontinued once the baby “sweats and refuses the kangaroo position” (Ruiz, 1998), signalling that the infant has matured enough to independently maintain physiological homeostasis (Charpak, 2005).
Methods of Evaluation & Impact of KMC Implementation
The impact of KMC has been studied extensively across a variety of settings. Traditionally, the primary focus of evaluation has centred around neonatal outcomes – specifically on its efficacy in reducing morbidity and mortality in LBW infants. However, despite the significant amount of studies that were available in the early years, there existed a paucity in the quality and relevancy of the literature, which inhibited its universal acceptance as a legitimate method. It wasn’t until 2011, when an updated and expanded Cochrane Review was published, that the method has been recognized as equivalent, and even superior to conventional neonatal care. This was corroborated by a variety of outcomes measured at several points in time.
The Cochrane study has most recently been updated in 2016, in which 21 randomized controlled trials (RCTs), including 3042 infants, were included. Out of the 21 RCTs that met the inclusion criteria, 19 evaluated the method in LBW infants after stabilization, one study evaluated it in infants before stabilization, and one compared early-onset KMC with late-onset KMC in relatively stable LBW infants. Further, 16 of the 21 evaluated intermittent KMC, and 5 evaluated continuous KMC (Conde-Agudelo & Díaz-Rossello, 2016).
While the trials were conducted under a variety of hospital conditions, regulations, and routines, the description of the employed KMC intervention was remarkably consistent across all trials, which was essential in establishing its positive impact. The studies analyzed various parameters in infants at discharge or at 40 to 41 weeks’ postmenstrual age, and at least one to three months’ follow-up. The findings concluded that KMC is associated with a reduction in mortality (RR 0.67), severe infections/sepsis (RR 0.5), and hypothermia (RR 0.28), as well as an increase in weight gain and incidence of breastfeeding both at discharge (RR 1.2) and at one to three months’ follow-up (RR 1.17) (Conde-Agudelo & Díaz-Rossello, 2016). It also concluded that there is growing evidence that KMC reduces the risk of nosocomial infection/sepsis, increases the gain in length and head circumference, increases maternal satisfaction with the method and increases maternal-infant attachment. Additionally, one of the studies in the review included that there were no significant differences between KMC infants and controls in a variety of neurodevelopment and neurosensory outcomes at one year of age (Charpak, 1997 and Conde-Agudelo & Díaz-Rossello, 2016). Numerical details of the findings can be referred to in Table 1 of the Appendix, which compares the outcomes of KMC versus those of conventional neonatal care.
As Cochrane Reviews are unofficially regarded as the “gold-standard” of systematic reviews of primary research in human health care, the endorsement offered through this publication has undoubtedly benefited the legitimacy of the impact of this method on a world-wide platform. This will likely serve to further assist its implementation around the world, as it concluded that the evidence is sufficient to support the use of KMC in stabilized LBW infants as an alternative to conventional neonatal care, particularly in low-resource settings. With all of this said, it is necessary to recognize that certain limitations do exist in the reporting of many of these studies. For example, the poor quality of a number of studies has left room for improvement, particularly due to the lack of blinding of outcome assessors and an unclear method of allocation concealment (Conde-Agudelo & Díaz-Rossello, 2016). This could have presented itself as reporting bias towards the KMC method. This most recent systematic review has also shown that more research is required to further substantiate the impact of the KMC method moving forward. For example, more studies are needed to investigate the long-term assessments of neurodevelopmental outcomes in KMC versus conventional-care infants, particularly regarding cognitive functioning, as only one such study exists. Moreover, there is a critical need for well-designed economic evaluations on the cost-effectiveness of the method in low-, middle-, and high-income settings. This will be further discussed in Part II.
***
Courtney Fang is in her final year at McGill pursuing a BSc. in Microbiology and Immunology and a minor in International Development Studies. She is interested in infectious disease control, increasing access to healthcare, as well as maternal and child health particularly in LMIC. Courtney plans to go to graduate school next year to pursue her Master’s in Public Health.
Zoë Goldstein is a 5th year Psychology student interested in the applications of global mental health programs. She is also interested in psycho-oncology and the emotional lives of all folks dealing with chronic conditions and illnesses. She intends to go to graduate school for clinical psychology.
Morgan Kennedy is in her final year of the MSc. Public Health program at McGill University with an option in global health. She is pursuing a career in public health knowledge translation for improved participatory research and policy change.
Carla Achcar is in her final year at McGill completing a BSc. with a major in Pharmacology and a minor in Biotechnology. She has great interest in the development of optimized diagnostic tools that hold the potential to democratize access to healthcare. She is actively engaged in global health organizations on campus.
Bidjinie Coriolan is in her final year of the MSc. Public Health program at McGill University. She has a great interest in maternal and child health as well as non-communicable diseases. She is pursuing a career in program management and policy design.
Mackenzie Hamilton is a 4th year Honours Immunology student at McGill University, studying the adaptive immune responses to influenza virus-like-particle vaccines. She is passionate about infectious disease control and improving access to necessary treatments in low and middle income countries. She plans on continuing her education in immunology and hopes to be able to translate this work to the global health field in the future.
Edited by Benjamin Aloi
References
Barros FC, Bhutta ZA, Batra M, Hansen TN, Victora CG, Rubens C.E. (2010). Global report on preterm birth and stillbirth (3 of 7): evidence for effectiveness of interventions. BMC Pregnancy and Childbirth 10:S3.
Bergh, A., Arsalo, I., Malan, A. F., Patrick, M., Pattinson, R. C., & Phillips, N. (2007). Measuring implementation progress in kangaroo mother care. Acta Paediatrica, 94(8), 1102-1108. doi:10.1111/j.1651-2227.2005.tb02052.x
Bergh A.M., De Graft-Johnson J., Khadka N., Om’Iniabohs A., Udani R., Pratomo H., De Leon-Mendoza S. (2016). The three waves in implementation of facility-based kangaroo mother care: A multi-country case study from Asia. BMC International Health and Human Rights 16.
Bergman N.J. (2015). Kangaroo Mother Care in African countries. Acta Paediatrica 104:1208-1210.
Broughton, E. I., Gomez, I., Sanchez, N., & Vindell, C. (2013). The cost-savings of implementing kangaroo mother care in Nicaragua. Rev Panam Salud Publica, 34(3), 176-182.
Cattaneo A, et al. (1998). Recommendations for the implementation of kangaroo mother care for low birthweight infants. Acta Paediatrica, 87:440-445.
Chan, G. J., Labar, A. S., Wall, S., & Atun, R. (2015). Kangaroo mother care: a systematic review of barriers and enablers. Bulletin of the World Health Organization, 94(2). doi:10.2471/blt.15.157818
Chan, G. J., Valsangkar, B., Kajeepeta, S., Boundy, E. O., & Wall, S. (2016). What is kangaroo mother care? Systematic review of the literature. Journal of global health, 6(1).
Chan, G., Bergelson, I., Smith, E. R., Skotnes, T., & Wall, S. (2017). Barriers and enablers of kangaroo mother care implementation from a health systems perspective: a systematic review. Health Policy and Planning, 32(10), 1466-1475. doi:10.1093/heapol/czx098
Charpak, N., Ruiz-Pelaez, J.G., Figueroa de, C. Z., & Charpak, Y. (2001). A randomized controlled trial of kangaroo mother care: results of follow-up at 1 year of corrected age. Pediatrics, 108(5), 1072-1079.
Charpak, N., Gabriel Ruiz, J., Zupan, J., Cattaneo, A., Figueroa, Z., Tessier, R., … & Mokhachane, M. (2005). Kangaroo mother care: 25 years after. Acta Paediatrica, 94(5), 514-522.
Charpak, N. & Ruiz-Peláez, J. G. (2007). Resistance to implementing Kangaroo Mother Care in developing countries, and proposed solutions. Acta Paediatrica, 95(5), 529-534. doi:10.1111/j.1651-2227.2006.tb02279.x
Charpak, N., Tessier, R., Ruiz, J. G., Hernandez, J. T., Uriza, F., Villegas, J., . . . Maldonado, D. (2017). Twenty-year Follow-up of Kangaroo Mother Care Versus Traditional Care. Pediatrics, 139(1). doi:10.1542/peds.2016-2063
Conde‐Agudelo, A., Belizán, J. M., & Diaz‐Rossello, J. (2016). Cochrane Review: Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Evidence‐Based Child Health: A Cochrane Review Journal, 7(2), 760-876
Darmstadt G.L., Bhutta Z.A., Cousens S., Adam T., Walker N., de Bernis L. (2005). Evidence-based, cost-effective interventions: how many newborn babies can we save? The Lancet 365:977-988.
Every Newborn: an action plan to end preventable deaths (2014). Geneva: World Health Organization.
The Global Financing Facility 2016-2017 Annual Report (2017). Washington, DC: World Bank Publications.
Hall, D., & Kirsten, G. (2008). Kangaroo Mother Care – a review. TME Transfusion Medicine, 18(2), 77-82.
Lawn J.E., Kerber K., Enweronu-Laryea C., Cousens S. (2010). 3.6 Million Neonatal Deaths—What Is Progressing and What Is Not? Seminars in Perinatology 34:371-386.
Lawn J.E., Mwansa-Kambafwile, Horta B.L., Barros F.C., & Cousens, S. (2010). ‘Kangaroo mother care’ to prevent neonatal deaths due to preterm birth complications. International journal of epidemiology.
Lima G., Quintero-Romero S., Cattaneo A. (2000). Feasibility, acceptability and cost of kangaroo mother care in Recife, Brazil. Ann Trop Paediatr 20:22-26.
Martinez, H. The Mother Kangaroo Method. Innovation for Development and South-South Cooperation (IDEASS Colombia).
Rasaily, R., Ganguly, K., Roy, M., Vani, S., Kharood, N., Kulkarni, R., … Kanugo, L. (2017). Community based kangaroo mother care for low birth weight babies: A pilot study. The Indian Journal of Medical Research, 145(1), 51.
Reaching the Every Newborn National 2020 Milestones: country progress, plans and moving forward. (2017). Geneva World Health Organization
Rosero, L., Martinez, R. (n.d.) Implementation of KMC in Colombian Social Security. (n.d.). Fundacion Canguro. Retrieved December 12, 2017, from http://fundacioncanguro.co/encuentros/2encuentro/abstract16.htm
Ruiz, J. G., (1998). Kangaroo mother versus traditional care for newborn infants 2000 grams: A randomized control trial. Journal of Clinical Epidemiology: Supplement 1, 51, S12-S12.
Ruiz, J. G., Charpak, N., & Cuervo, L. G. (2004). Kangaroo Mother Care, an example to follow from developing countries. BMJ : British Medical Journal, 329(7475), 1179–1181.
Ruiz, J. G., Charpak, N., Castillo, M., Bernal, A., Ríos, J., Trujillo, T., & Córdoba, M. A. (2017). Latin American Clinical Epidemiology Network Series – Paper 4: Economic evaluation of Kangaroo Mother Care: cost utility analysis of results from a randomized controlled trial conducted in Bogotá. J Clin Epidemiol, 86 (Supplement C), 91-100. doi:https://doi.org/10.1016/j.jclinepi.2016.10.007
Seidman, G., Unnikrishnan, S., Kenny, E., Myslinski, S., Cairns-Smith, S., Mulligan, B., & Engmann, C. (2015). Barriers and Enablers of Kangaroo Mother Care Practice: A Systematic Review. PLoS ONE, 10(5), e0125643. http://doi.org.proxy3.library.mcgill.ca/10.1371/journal.pone.0125643
Sharma, D., Murki, S., & Oleti, T. P. (2017). Study comparing “Kangaroo Ward Care” with “Intermediate Intensive Care” for improving the growth outcome and cost effectiveness: randomized control trial. J Matern Fetal Neonatal Med, 1-8. doi:10.1080/14767058.2017.1359832
Shrestha, M. & Pokharel, N. (2005). Hypothermia in newborn babies. The Nursing Journal of India, 96(11), 255-256.
Simkiss, D. E. (1999). Kangaroo Mother Care. Journal of tropical pediatrics, 45(4), 192-194.
Wardlaw, Zupan, Ahman (2004). Low Birthweight: Country, Regional and Global Estimates. UNICEF.
World Health Organization. (2017). Preterm birth.
World Health Organization. (2003). Kangaroo mother care: a practical guide.
